Is Fasciotomy for You?
from
Dean Ripa
on
January 11, 2011
Add a comment about this article!
Dean Ripa was probably the first researcher to speak out formally against
fasciotomy in snakebite treatment. With his permission, I have reproduced a
chapter from his book, The Bushmasters (Genus Lachesis, Daudin 1803):
Morphology in Evolution and Behavior. This work was first published on CD-
Rom over a decade ago, and written during the 1990s. This is from a revised
version published in 2001. Dean’s statements on this subject have had wide
influence peripherally, transmitted through the snake world as though by
osmosis, although with few persons realizing where the source actually
originated. I have included the entire chapter as it sets the stage for his
discourse on the evils of invasive surgery in snakebite, something physicians
should pay close attention to. To any one who works with dangerous snakes, this
chapter should be committed to memory—or perhaps carried with you into the
medical treatment room. –Tom Chaudoin
Is fasciotomy for you?
Bite by Lachesis or Bothrops –Who’s who? Muscle necrosis – is
surgery warranted? Origins of snakebite treatment: therapeutic exorcism?
by Dean Ripa
Copyright 2001;2003
IF THERE IS A MORE INEXACT STUDY than
the demographics of snakebite, I can’t
imagine what
it could be. You start out with a panic
stricken victim
who knows only some folk names for snakes and
may not even have seen what creature bit him,
and
you finish with a doctor whose own set of
folk names
may not even coincide. If the victim dies, it
must have
been one of the “deadly” ones; if he lives,
it must have
been one of the “less dangerous” ones—and to
that
end the bite shows up in the records. Next
you have
“official sources” who may not even be in the
health
care business, lumping the accident in among
all the
other fatal intoxications in the region, from
food poisoning
to drug overdose. At last comes the snakebite
* To cite this article: Ripa, D. 2003. Is
fasciotomy for you?
in The Bushmasters (Genus Lachesis Daudin,
1803) Morphology
in Evolution and Behavior; 3rd Edition.
Electronic
book. Cape Fear Serpentarium. Wilmington, NC.
specialist from overseas who is has in mind
to publish
a paper—after that, you can smell the data
cooking!
Meanwhile, the snake is still in the woods
and has not
said a word about it. Of those involved, he
is the
wiser.
So I feel a little uneasy quoting the latest
projections
on world snakebite, with their fine details
of incidence,
morbidity, lethality and mortality, all
neatly
divided up from the real mishmash. The most
venomous
species get blamed over and over, while their
not-so deadly cousins are repeatedly
exonerated.
Once in a while a so-called “positive ID” is
made,
although you never quite understand how the
mere
addition of a little protein in people’s
veins can produce
so profound an improvement upon their
recognition
skills. It will take some coaching above the
hospital bed to reduce the size of the
villain to feasible
�
proportions. While the folk names swing back
and
forth giddily between surviving family and
friends, and
the debacle begins as to what laid little
Pedro to rest,
or sent old Guilherme to the big expensive
hospital in
the city, two culprits rear their poisonous
heads: a
snake of unknown kind and size, and a doctor,
who
may not have had the least idea how to treat
the case.
Some doctors, of course, really do save
lives,
and some victims know exactly which snakes
have
bitten them. Nevertheless, this percentage is
probably
not very high in tropical countries. In the
Old World
with its kraits, mambas and cobras this is a
more serious
issue than in the Americas, owing to the
delayed
effect of some envenomings which may resemble
harmless snakebites almost till the very end.
In these
cases, the formula is usually to wait for
symptoms.
But waiting on symptoms in a krait bite is
like waiting
for the coroner; by the time the typical
breathing difficulties
appear, it may be too late to alter the
course.
Then there is “delayed presentation,” a
problem occurring
pretty much everywhere there are snakebites.
The case may be three days into gangrene
before the
doctor sees it, putting the initial symptoms
so far along
that what began as a battle against a deadly
venom is
now a war against an even deadlier bacteria.
Home-
remedies ranging from tourniquets to
poisonous leaves
add to the melee. Assuming none of this
happens,
that all the right things are in place—smart
doctor,
early presentation, and good, clearly
diagnosable
symptoms—then one can start picking out an
anti-
venom. Polyvalent serums can simplify
treatment, at
least regionally, so in some instances this
seems hardly
important. However, the bites of certain
species
require special attention, not only as
regards the type
of antivenom to use (e.g., “neurotoxic”
crotalids in
the Americas), but in the whole therapeutic
approach.
Genus Lachesis is one of these, and confusion
with
the more common and similarly colored
Bothrops
could make a significant difference with the
approach
to treatment. Fortunately, some variations in
early
presentations exist. In this section I review
bushmaster
morbidity, and compare envenomings with its
more
common congeners, showing ways for
distinguishing
between the bites when the snake has not been
seen
or has possibly been misidentified.
Statistically, bushmaster bite shows a low
morbidity,
but high mortality in all parts of its range
(Bolaños,
1982; Gutiérrez et al., 1995; Hardy and
Silva, 1998).
By contrast, terciopelo (Bothrops asper) bite
shows a
low mortality, but (as with all Bothrops) an
overwhelmingly
greater bite incidence (Gutiérrez et al.,
1980). Yet
there is a disparity, for as Hardy and Silva
(1998) note,
“…venom yields and LD50s from the laboratory
suggest
that the terciopelo is potentially more
lethal than
the matabuey [bushmaster, L. stenophrys] in
terms of
an individual human envenoming…[The
bushmaster
has] a proportionally smaller head and venom
gland
(pers. obs), smaller initial venom yield (233
mg)…[lower] maximum yield of 407 mg (Da Silva
et
al., 1989) and lower i.v. venom toxicity for
laboratory
mice (LD50 5.6 µg/g in mice).”* Contrasting
the very
high mortality rate of bushmaster bite to the
significantly
lower mortality rate of the terciopelo, the
authors
conclude, “The lesson to be learned is that
mice are
not human beings. The variation in
susceptibility to snake
venoms makes extrapolation of lethal doses
from one
species to another an exercise in futility.”
The truth is that if we compared the LD50s of
the
majority of snakes with the medical data, we
would
find that bushmasters were not so unusual in
this regard.
Numerous snake species frequently implicated
in fatality would be determined in the
laboratory to be
unequipped to do so; while some for which
fatality
records were rare would be deemed gravely
venomous
(Chapters 24 - 25). But the medical record is
distorted by its own artifacts.
Chapter 5 (and Table 8) explores the sizes
attained
by Bothrops species and shows that at least
one of
them, the terciopelo (B. asper) is quite
similar to
Lachesis in length and may even outstrip it
in modern
Central America. Large female B. asper reach
2 m or
greater, are not rare snakes, and in any
event, much
more often encountered than bushmasters by
native
people. The really big Bothrops are soon
killed out
from agricultural areas, leaving smaller
examples to assume
their place reproductively. No matter, the
dimensions
of the venomous apparatus remain nearly the
same. The head-size (venom gland and fang
size) of
an adult female B. asper, at 1.7 m length, is
not much less than that of a specimen of 2 meters, and
capable
of expending huge amounts of venom in a bite.
The
really big terciopelos (> 2 m in length)
occur mostly in
secondary forest situations (cohabited by
occasional
bushmasters), around small farms, and not
near the modern
mass agricultural projects where snakebite is
less
common. As with bushmasters, these larger
adult individuals
likely account for the minority of bites.
They are more conspicuous, easily avoided, and
live in more
remote situations
*L. stenophrys 5.5 mg/kg i.v. and 6.2 mg/kg
intraperitoneally (i.p.) (Bolaños, 1971); 95
µg i.v. (5.6 µg/g) and 110.5 µg i.p. (6.5 µg/
g) in 16-18 g mice (Bolaños, 1972) and 112 µg
(6.6 µg/g) in 16-18 g mice; and for L.
melanocephala 8.9 µg/g. For L. stenophrys
in Colombia 9.8 µg/g (Bolaños et al., 1978),
and 6.8 µg/g for L. stenophrys from the
Pacific Coast of Colombia (Otero et al.,
1992).
For L. melanocephala the LD 50 was 103 µg
i.p. (6 µg/g) in 16-18 g mice (Gutiérrez et
al., 1987).
�
This brings us to our first artifact.
Statistics attempt
to implicate species in snakebite morbidity,
but they
almost never record the size (or at least an
accurate
size) of the individual specimen involved.
While bushmasters
and terciopelos are comparably large snakes,
the lower mortality for the terciopelo (than
bushmaster)
may be due in great part to the generally
large average
size of the bushmasters that usually bite
humans,
these being almost entirely adult snakes,
while the terciopelos
involved in snakebite are almost entirely
examples
of small size, usually juveniles or neonates.
But
this has nothing to do with the potential of
Bothrops to
reach large size, for these are at least as
common, if not
more common, than the large Lachesis. It has
to do
with the extraordinary reproductive potential
of Bothrops,
where at any given time babies and juveniles
outnumber
adults.
Fecundity and snakebite
The average wild-caught bushmaster measures
almost
exactly 2 meters. Bushmasters are found so
exclusively
at this size that hunters, collectors, and
wildlife
dealers consider finding smaller ones a rare
event,
while the odds of finding a baby bushmaster
is probably
less than one in twenty adults (Chapter 5).
Since
finding even an adult bushmaster is a rare
thing, this
puts babies in an even more remote category.
Fittingly,
envenomings by baby bushmasters are almost
unknown
in the literature. Torres et al. (1995)
mention a single
case of the bite of a “juvenile” snake, but
this specimen
is of unspecified size and age. Prior to my
own bites
recorded in Chapter 22, bites by truthfully
“baby” bushmasters
had never been recorded. Thus the encounter
rate reflect almost entirely bites by adult
examples, and
with almost none at all by the neonate. But
we have a
disparity, for in Bothrops this is quite the
reverse. Here
neonate and juvenile bites outnumber those of
adults
by many, many times.
This is easy to prove, both from personal
interviews
with the bite victims, and from the treatment
data itself,
where the sizes of the snakes can to some
extent be
inferred by the anatomical placement of the
bites. In
Costa Rica (probably the country best
documented),
about 50 percent of all bites occur on the
bare feet,
and 32 percent on the upper extremities,
mostly the
hands (Bolaños, 1982; Gutiérrez et al.,
1995). People
step on the snakes bare footed, or
accidentally
put their hands on them. These snakes are
undoubtedly
small examples whose inconspicuous size has
rendered them unseen. The majority of these
accidents
are believed to involve Bothrops, and as
these
are most populous, this is reasonable.
Without, however,
implicating the probable sizes of these
Bothrops
as yet (but see below), let’s compare these
with
accidents involving bushmasters, whose body
length
we can almost always assume to be in the 2 m
range.
Here the clinical data suggests a different
anatomical
site than that involvingBothrops, primarily
involving
the lower limb, but not the feet. Bushmasters
bite
higher up on the body (knees, calves, ankles,
etc.,)
resulting from a long striking range and
great body
length. It is reasonable that large Bothrops
would
follow this example, and strike high. With
only 18
percent of all bites on the legs above the
feet, we can
conclude that this percentile does not
involve neonates
and juveniles. Therefore, large adult
Bothrops
bite people not more than about 18 percent of
the
time. This puts them in the least category of
bite incidence,
while the greater, 82 percent, involve their
smaller conspecifics. Deductively then, we
can reason
that about 82 percent of all snakebites in
Latin
America (50 percent foot bites and 32 percent
hand
bites) are caused by snakes smaller than the
average-
sized bushmaster (or adult terciopelo) of 2 m
length.
How curious that baby bushmasters never bite
anybody,
but that baby Bothrops bite the most people
of
all! Indeed, it is the baby, not the adult
Bothrops that
are causing the overwhelming majority of
snakebites!
What makes this so? The answer lies in the
remoteness
of the habit where baby bushmasters are
hatched,
and the incredible fecundity of Bothrops,
which deliver
their enormous litters of fifty or more
living young
near human traffic. During the first months
of the birth
season, which occurs in September through
December
(Solórzano and Cerdas, 1989; and pers. obs),
a
hectare of reclaimed agricultural land could
be inherited
by literally hundreds of neonatal Bothrops,
with
only two or three adult females necessary to
produce
this number. Most of these babies will not
survive to
become adults; nevertheless, they will
survive long
enough to plague snakebite statistics. The
records are therefore much biased with the bites of
these smaller,
inconspicuous, and more numerous babies.
Bites
by their much less populous parents are
logically in
the minority, vastly exceeded in number by
the younger,
smaller snakes.
We can predict less severity in the bites of
smaller
snakes than large. Bites by baby Bothrops
should
seldom be fatal to adult humans, even without
treatment;
on the contrary, the bites of large Bothrops
should often be fatal to adults even with
treatment.
So this is a strong artifact affecting our
comparison.
We can predict that highly fecund species
like Bothrops
will figure more extensively in snakebite
statistics
than those whose recruitment rate is less
prodigious;
further, that bites by the less venomous but
more
numerous juveniles of these species will
always be in
the great majority. In Africa we should see a
similar
corollary with Bitis, where bites by the very
prolific
B. arietans and B. gabonica, for example,
will again
reflect statistics gathered after the bites
of baby or
young snakes, most of the time. This accounts
for the
lower than expected mortality rate from
envenomings
of these formidably armed species, and others
of their
ilk.
There are other artifacts. Bothrops has a
strong
sexual size dimorphism, producing a
dramatically
smaller male with a much smaller head (i.e.,
less venom
and shorter fangs), than the female. Even
when
the adult male Bothrops totals equal length
with the
female, the male will be less than half her
mass. The
diminutive male is more commonly encountered
than
the larger female, by about 2:1 (my
collecting data).
The degree to which the drastically smaller
male (than
the female) figures in snakebite incidence is
certainly
unknown. Yet we can assume that bites by the
smaller
males occupy the greater portion of the 82
percentile
of foot bites and hand bites, their smaller
size
making them difficult to see and avoid, than
females.
With their lower encounter rate, the larger
females
should (or could) be culled primarily from
the 18 percent
bites to the lower limb above the foot. The
bites
from larger snakes should then be the most
often fatal.
This is important, for we begin to see that
very
grave or rapidly fatal Bothrops syndrome (of
bleeding
to death despite treatment), is a female
biased
equation. The male, being less than half the
mass of
the female, and with its dimorphically much
smaller
head (and venom glands and fangs), will be
the less
venomous of the sexes.
Bites by large snakes are potentially more
severe
than bites by smaller snakes of the same
species, owing,to a larger volume of venom and longer fangs.
A subcutaneously
administered Bothrops toxin is dramatically
less potent than an intramuscularly injected
one,
and if administered by a neonate in
proportion to its
available venom, perhaps could not even kill
an adult
human being (Chapters 24 - 25). Death from
Bothrops
bite in adult human beings should, then,
always
require the necessary fang length to permit
intramuscular/
intravenous inoculation. Based on venom yield
and
laboratory toxicity, it seems probable that
if bites by 2
m long Bothrops predominated (as they do for
Lachesis) the mortality rate would be much
higher than
now. Extrapolated from tests on rodents (but
we cannot
vouch for this accuracy in humans), a large
terciopelo
possesses enough venom to kill 20 or more
people
if injected by the intramuscular route (up to
1530
mg; Bolaños, 1982). Its fangs are even longer
and
stouter than the bushmaster’s; indeed, B.
asper has the
longest fangs of any snake in the world,
exceeding 3
cm in large specimens (usurping Bitis
gabonica from
that honor; see Chapter 11). The chance of
these formidable
weapons striking an important blood vessel is
as great as in the bushmaster, and
intramuscular injection
is assured. When snake-size is equal, the
fatality
rate for Bothrops bite should as high as
Lachesis bite.
The higher than expected survival rate for
Bothrops
envenoming is a statistical effect, and not
the least bit
factual when applied to bites by large
females. It is
skewed by a preponderance of bites by
immature
snakes (ca. 50 juveniles to one adult female
born each
year; thus 50:1), and of the dimorphically
smaller males
(conceivably > 2:1 females). As such, when we
talk of
Bothrops bites and compare lethality to other
species
like bushmasters, our terms are not
sufficiently descriptive.
Factually speaking, we are not talking about
a
single type of bite at all. So different are
the venomous
capabilities of juveniles, males and females,
it is as
though we were not even talking about the
same species.
What’s in a name?
Names don’t mean much in the backwaters of
the
tropical world. Here the snakes are merely
actors in a
hereditary drama where the biggest species
get first
billing and the most credit for killing the
patrons. Local
monikers like matabuey, cascabel muda,
surucucu,
makasneki, and verrugosa, etc., answer for
any large-
headed, rough-scaled serpent that is not the
familiar
boa constrictor and has a reputation for
mayhem. The
woods may be full of terciopelos, but the
largest terciopelos are, by some marvelous conversion,
bushmasters.
Size is the native standard by which the
names
for bushmasters are applied—and misapplied.
The
scientist not taking this problem into
account will make
more of local names than is their due, and
impose an
even greater sense of disorder upon his
statistics.
If most terciopelo bites are by baby or young
snakes,
a bite by a neonate bushmaster cannot be
substantiated
by a single verifiable case. The literature
describes
an envenoming by something vaguely called a
“juvenile”
(in Torres et al., 1995), but this would seem
to
cover a broad area of possible dimensions:
what is a
“baby” and what is a “juvenile” in relation
to snake-age
and snake-size? Subjectively speaking, a
neonate could
be anything from 1 day to 6 months old,
depending on
the reporter’s whim. A juvenile could be all
these,
more than a year old and a meter long. This
size difference
would have profound consequences on the
recorded
severity of the bites. If neonate and
subadult (<
ca. 100 cm) bushmasters were included in the
statistics
to the extent of neonate and subadult (< ca.
100 cm)
terciopelos, what would be the result?
Certainly we
would see fewer bites involving the lower
limbs (which
comprise most non-interactive bushmaster
bites to date)
and more bites involving the feet and hands.
In all likelihood,
however, misidentification would prevent
these
examples being called “bushmasters” to begin
with. The
local vernacular would connect them with
several typically
smaller, more familiar species, and not with
Lachesis.
Bushmasters are nowhere plentiful, but none
less
than the almost supernaturally rare babies.
Offering a
bounty in Costa Rica, Panamá, Suriname,
Ecuador, and
Brazil, I observed this mystery first hand.
Once in a
while the native catchers would bring in a
fairly young
example (< 40 cm), but in no case a newborn
bushmaster
prior to its first skin shed; nor all my
years tramping
through bushmaster habitat was I ever blessed
by
an encounter with a baby bushmaster myself.
Such
young specimens (> ca. 6 months) as were
brought in
were remarkably few, always outnumbered by
the perennial
2 meter adults. Nearly all specimens were
found
in forest that was being slashed for
agriculture. By contrast,
approximately 20 baby/juvenile (< ca. 100 cm)
B. asper were taken, to every one large (>
1.6 meter)
adult of that species, these numbers
snowballing in the
birth season. As in collecting, where it is
the large, 2meter-
long adult bushmaster that is most often
encounttered,
it is the adult bushmaster bite that has most
often
found its way into snakebite statistics, to
the extent
that it dominates all others. Even if newborn
or small
bushmasters did often bite people, and were
abundant
in agricultural areas and near human
dwellings like
terciopelos, the chances of them being
described statistically,
is small. The tendency would be to absorb
these bites into the greater morbidity of
Bothrops and
related genera. For example, the little
tamaga (Porthidium
nasutum) makes such a convincing “baby
bushmaster” that most of my collectors could
not tell
the difference even after I had provided them
with
photographs. This proved true in all regions
where
Lachesis overlapped with Atropoides nummifer,
as
well; and even in regions where they did not
overlap,
owing to the transient human populations who
had
experience with them. Jumping vipers became
bushmasters
when bushmasters were more than about 1
meter’s length.
Rural doctors are not well educated to tell
the difference
either, confusing Bothrops (asper, atrox,
etc.)
with other venomous ground vipers as a matter
of
course. Whether the bite is by one of the
Porthidium
species, or any other potentially less
venomous kind,
the easy path is to blame it on the better
known terciopelo
(or other Bothrops taxa). As with the
bushmaster,
few rural Costa Ricans bother to distinguish
between the much less venomous tamaga (P.
nasutum)
and the terciopelo. One is simply the “baby”
of
the other. Hence an enormous number of bites
attributed
to the terciopelo may in fact involve the
little
tamaga.
There are other confusions of size. For
example,
when a terciopelo reaches about 2 meters in
length it
automatically becomes a matabuey in the
popular
mind. It can do what its name implies—kill an
ox—
so why not? Matabuey (ox killer) and cascabel
muda (silent rattler), although names
intended for
bushmasters, means a viper of large
proportions, little
more. For example, when I put out a bounty
for
live matabuey in rural areas near primary
forest, I was
disappointed to receive almost all large
terciopelos
(>1.5 m) until my catchers (and in turn their
catchers,
for they were quick to make a business of it)
learned
to tell the difference. Hence, to be bitten
by a large
terciopelo was to be bitten by a matabuey, as
far as
the local people were concerned. With inverse
logic,
bites by baby bushmasters would probably have
been
blamed on terciopelos (or else on tamagas,
which is
what the few baby bushmasters brought to me
by
native collectors were typically called), had
any occurred.
In effect, to many residents there were no
small bushmasters, only terciopelos, just as
there were
no large terciopelos, only matabuey. I have
encountered
similar phenomena in all parts of the
bushmaster’s
range. Even in mainland South America local �
collectors confused the smaller Bothrops
atrox with
bushmasters, once the Bothrops exceeded a
certain
size.
Identification through symptoms
All this reflects statistically when doctors
start asking
their patients what bit them. They may be
left with
only the symptoms to identify the culprit,
and yet building
a picture of snakebite according to this sort
of
diagnosis is a haphazard affair, for the
treatment protocols
for bushmaster are very different. In the
next
pages I devise a workable diagnostics based
on visible
alterations easily seen on presentation, and
that
will hopefully make treatment simpler and
more successful.
In cases of severe envenoming, differential
diagnosis
of Lachesis with Bothrops can be summed up
by two words: shock and hemorrhage. If the
victim
presents skin blistering or blackening of
local tissue,
or any systemic hemorrhagic sequelae within a
short
time frame (ca. 5 hours) after the bite, the
culprit is
Bothrops and not bushmaster. Reports in
literature,
TV nature programming, etc., of bushmaster
bites
causing “bleeding from eyes, nose and mouth”
are
undoubtedly based on misidentification by
resident
persons. However, systemic alterations such
as early
shock (i.e., hypovolemia) are definite signs
of bushmaster
envenoming. Although there is no doubt that
the bite of a large terciopelo could produce
shock
effects analogous and as severe, these would
likely
be delayed and already accompanied by some
visible
blood incoagulability and/or early skin
necrosis.
Indeed, posing so severe an envenoming from
Bothrops
that it would produce the rapid systemic
alterations
of bushmaster bite is to pose concomitant
hemorrhage,
with extravasation, thrombocytopenia,
multiple
local hematomas, and systemic hemostatic
disorders
including mucosal bleeding (e.g., epistaxis),
hemathidrosis, occult bleeding in the GI and
GU tracts
(presenting as hematamesis, hematochezia,
urticaria,
&c.), and in severe cases, deep visceral
hemarthrosis.
Renal and hepatic bleeding and even cerebral
hemorrhage are an expected prognosis. In
bushmaster bite, the patient would already have died
from
shock before these delayed effects could take
place.
If he were not experiencing severe shock to
go with
his free-bleeding, then it would not be a
bushmaster
that had bitten him (see descriptions of
bushmaster
bite shock, i.e., Lachesis-syndrome in
Chapter 22).
The effects of bleeding to death can be seen
on
the small scale in the edematous area
surrounding the
puncture wounds in Bothrops. The latter will
turn
quickly black, making a blood-blister. Blood
and
serum filled bullae will appear on the bitten
extremity
within as little as 2 to 4 hours and usually
before 12
hours (Fan and Cardoso, 1995; and pers. obs).
This
blistering may advance over the course of
days, reaching
large size. But there is little or no
blistering in
bushmaster bite. In the Bothrops bite the
patient may
feel the skin “stinging with fire” throughout
the extremity,
and be unable to distinguish this feeling
from
that of an actual fire burn; however, in
bushmaster
bite, while there is a feeling of a
germinating fire (initially),
the oncoming sensations of “having one’s limb
plunged into boiling oil” may be absent.
Bushmaster
bite pain is primarily like that of blunt
trauma; a concentrated,
heavy, pounding ache, emanating from
within the muscle and tendons, rather as
though one
had shut ones hand in a car door and were
repeating
this operation till a sense of near numbness
supervened
in tissue no longer equipped to feel
anything.
Or, if into deep muscle, the pain may take
the feeling
of impalement, as of a sharp dagger plunged
through
the limb and being twisted back and forth.
The pain
is mindboggling, and may be so severe that
the victim’s
teeth chatter and his whole body jumps
convulsively.
For all that, the feeling of fire-burn is
mostly
absent, probably from the venom being less
hemorrhagic.
The pain dulls down after some days to a
crashing repetitive throb, and you can
tolerate it. In
Bothrops, however, the fiery pain is
continuous, and
feeling of “flames” dancing transiently about
the limb
in areas remote from the inoculation site,
may persist
for more than 6 weeks. In Bothrops bite, the
fang
punctures will always turn black, and if
presenting as
dark blue or purple will soon turn black,
while the
bite wounds and/or surrounding areas will
blister. In
bushmaster bite the wounds may appear darkly
* Bushmaster envenoming produces some of the
most extreme edema of any snake species. I
have endured swelling so tense
that even to twitch the fingers or elbow was
to cause the skin to split open.
Nevertheless, I believe fasciotomy to relieve
compartmental pressure is never indicated in
these or any other species. It causes
permanent scarring, increases likelihood of
infection and advances necrosis. Moreover, it
prolongs and exacerbates deadly shock. The
dangers of compartment syndrome
are wildly exaggerated. Watt (1989) notes,
“Tense edema in the bitten limb rarely leads
to vascular compromise.”
�
bruised, but they are basically clear and
will not necrotize
(but if any necrosis occurs at all, it will
likely
be here). Exorbitant edema may give the skin
an appearance
of near bursting.* If blood escapes beneath
the skin surface (extravasation), it will be
due mostly
to the pressure of the swelling rather than
from the
degradation of the blood vessels by the
venom. If
sufficient antivenom is given soon enough
there should
develop little or no skin discoloration other
than bruising.
Not so with a Bothrops bite, where the blood
from ruptured blood vessels always turns
black, having
hemorrhagic or necrotic contents. The fang
wounds in the bushmaster bite may cease
bleeding
within a few minutes of the inoculation, the
pressure
of the swelling literally closing the wounds
shut, although
there may occur a clear serous discharge.
With
prompt and sufficient antivenom the fang
wounds will
rarely abscess, except from secondary
contamination.
In the Bothrops bite, the fang wounds will
turn
black regardless of antivenom treatment and
will almost
always abscess with bloody pockets of
hemorrhagic
cellular debris regardless of infection. Note
that a “venom abscess” reflects the
hemorrhagic properties
of the venom and is distinct from a bacterial
abscess, but both may occur in concord. A
scorched-
looking, blackened limb covered with bullae
and growing
hard with necrosis is not from the bite of
the bushmaster.
It is the signature of the Bothrops.
Silva (1980/81) made the first attempts to
differentiate
these symptoms diagnostically. His
conclusions
reflect bites by Lachesis muta muta so they
may differ somewhat from my first hand
reports of
bites by the Central American species, with
respect
to skin necrosis (perhaps greater in L. muta
muta
although still milder than in Bothrops);
however, the
systemic effect remains remarkably similar.
Cardiovascular
changes occur within 15 minutes of the
accident,
with severe hypotension, bradycardia, blurred
vision, intense abdominal pain, colic,
diarrhea, and
vomiting before 1 hour. In Bothrops, he
concludes,
the hypotension occurs much later, 10 hours
or more
after the accident. And as I have reported in
the previous
chapter, hemorrhagic effects are much more
intense
in Bothrops and may be altogether lacking in
Lachesis.
As noted from my own bite experiences, a
distinction
should be made about the “abdominal pain”
syndrome associated with bushmaster bite.
This has
been attributed, wrongly, I believe, to colic
and diarrhea.
Although the latter occurs in consort, the
stabbing
pains are not gastric in origin. If they are
not actually nerve-related (e.g., from vagal
stimulation),
they are more nearly distributive, related to
hypovolemia.
This thronging, convulsive, and altogether
unique agony is peculiar to what I have
dubbed the
“Lachesis-syndrome.” Chapters 25 - 26 explore
new
data and pursues this theme further.
Muscle necrosis—is surgery warranted?
Muscle necrosis has been reported in
bushmaster
bite, historically in a review of four cases
of L.
stenophrys bite in Costa Rica (Bolaños,
1982); in a
case of L. muta muta bite in Colombia (Hardy
and
Silva, 1997); and more recently in an
interactive bite
involving a professiona snake-catcher, also
in Costa
Rica. In all cases the muscle necrosis was
encountered
during fasciotomy-incision, relatively soon
after
the bite (within four days). In all cases the
muscle
necrosis was described as “extensive.” All
patients
received varying amounts of antivenom
therapy, however,
in the Colombian case antivenom was given
sparingly, and long after the bite occurred.
In this
section I review these cases, and compare
them to
my own bites and some others. I review the
effects
of surgery in bushmaster envenomations, and
conclude
overwhelmingly that it causes serious deficit
and
leads to death in early treated cases.
Gutiérrez et al. (1990) notes in laboratory
tests on
mice “abundant erythrocytes and mild
myonecrosis
in muscle injected with venoms of adult, two-
year old
and one-year old specimens of L. stenophrys.”
In
these cases, there were abundant erythrocytes
in the
interstitial space and a relatively small
amount of necrotic
muscle cells.” In other words, the necrosis,
however mild, was always located in areas of
abundant
hemorrhage. Granting that venom
susceptibility
in human beings may be different than in
mice, the
resemblance between hemorrhagic cellular
debris to
necrosis is certainly striking. Both appear
black (or
very dark) in color, indurate, and certainly
constitute
an accumulation of “dead” material. This
could provide
a convincing mimic of necrosis to physicians
unaccustomed
to seeing it, and in the resulting anoxia
caused by surgery, catch more than a little
blame for
what it is due. In extremely edematous tissue
such a
mock necrosis could appear extensive,
especially
where hemorrhage has been increased by
surgery.
Significantly, in the five envenomings
described in
Chapter 22, neither muscle nor skin necrosis
occurred.
In ten envenomings in Souza and Buhrnheim
(1995),
�
necrosis was not a problem. Given these
disparities,
we can at least concede that a large window
of uncertainty
exists for an accurate diagnosis of muscle
necrosis in bushmaster bite cases.
As such, myonecrosis in promptly treated
bushmaster
bite might be either: (1) confusion with
Bothrops
bite, where the long fangs of the Bothrops
have
delivered the potent myotoxin deep into
muscle; (2)
misdiagnosis based on confusion with
erythrocytic
debris in the muscle cell interstices (sensu
Gutiérrez
et al., [1990]); (3) tissue anoxia from
hemorrhage
started by the surgical procedure; or (4)
actual myonecrosis.
I strongly suspect that the majority of all
early treated
bushmaster bites, where sufficient antivenom
is given
and severe skin and muscle necrosis is
reported,
are either cases of misidentification of the
snake (e.g.,
it was really a Bothrops species), examples
of tissue
anoxia resulting from secondary infection
and/or increased
hemorrhage enhanced by surgery (fasciotomy,
excision, &c.,) and/or confusion with
existing
erythrocytic debris also enhanced by surgery.
Any of
these local alterations could convincingly
impersonate
muscle necrosis to physicians inexperienced
with the effects of snakebite (as most are);
especially those
physicians persuaded by medical literature to
expect
myonecrosis in envenomings by all large
vipers.
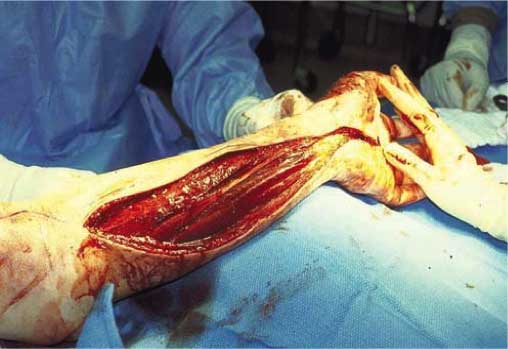
Fasciotomy after bite from a captive Crotalus
oreganus helleri.
Figure 2. Intracompartmental pressure is
measured in
the arm.
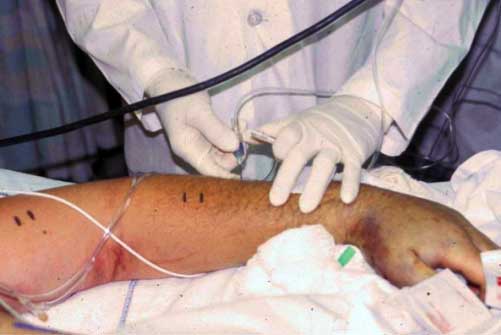
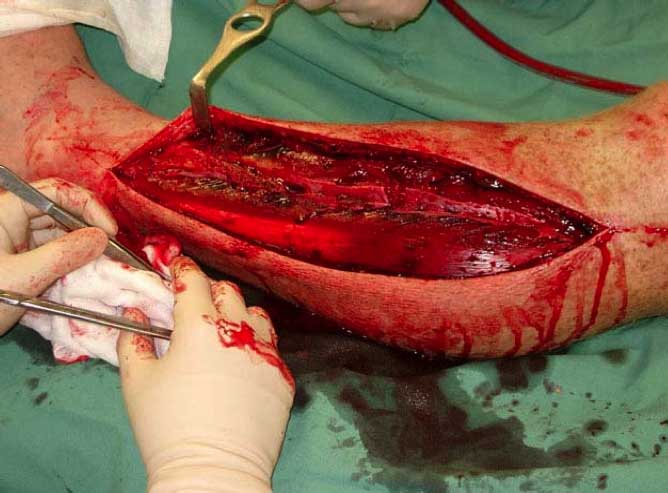
Figure 3. Intraoperative view of
fasciotomy.
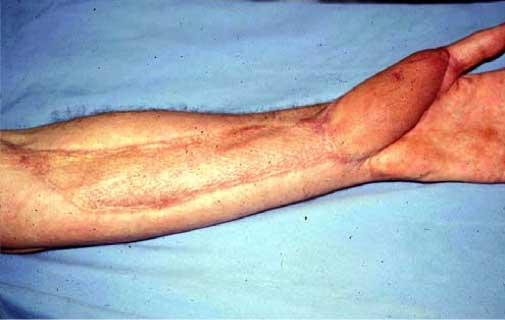
Figure
4. Three years post-bite after skin grafts
and muscle
transfer. Photos Robert Norris.
Perhaps medical literature has used the term
“myonecrosis”
too liberally, not only in regard to
bushmaster
bite, but in many other snakebites, as well.
Russell (1983) remarks on the rare occurrence
of
necrosis in the North American crotalid
envenomings
he has treated; and I would suppose all of
these to
possess more strongly necrotizing venoms than
Lachesis. Fan and Cardoso (1995) note the
occurrence
of necrosis in less than 10 percent of
Bothrops
envenomings; and in laboratory tests on mice,
the
venom of Bothrops has been shown to have a
more
necrotic action than that of Lachesis
(Gutiérrez et al.,
[1990, 1980], Rucavado et al., 1999). Yet
some
recent literature on bushmaster bite would
have us
believe that muscle necrosis occurs in a
majority of
cases.
Consider the ethical justifications in a
medical profession
already determined to use surgery for other
reasons (e.g., to prevent or relieve a
suspected “compartment
syndrome; but see below). An averred
“muscle necrosis” expiates the damage caused
by
surgery, and supports the importance of
surgery as a
valid means of resolving an always uncertain
condition.
A diagnosed “muscle necrosis” can always be
dragged out after the fact even though the
surgery
itself may have encouraged its development.
It is not
unexpected that inaccurate or misleading
medical reports
should find their way into the medical
statistics,
�
giving the impression that myonecrosis is
rather more
common in snakebite than it actually is.
Sadly, this
may have resulted in many unnecessary
surgeries,
keeping this expensive and damaging procedure
in
use as a standard practice. Ultimately,
however, the
debate over muscle necrosis is less important
than
the radical methods chosen to deal with it,
and vitally,
the time-period during when these selected
methods
are applied. It is this critical time-period
that will have
most to do with whether the patient survives
of not to
pay the medical bill.
Bear in mind that surgery is not usually
elected to
correct some unseen necrosis whose existence
the
physician might suspect, but cannot really
determine,
before opening the bitten extremity. The
initial surgery
is usually performed to relieve edema. This
technique,
called fasciotomy, attempts to sever the
constricting
band of the fascia which, with gross
swelling,
might cut off the blood supply to the
extremity (or is
so feared). The fascia, unable to expand with
the
swelling, becomes a sort of inner tourniquet.
Fasciotomy
provides an opportunity for other sympathetic
invasions afterwards, such as surgical
debridement
and excision. It gives the physician a chance
to
see what horrors may be stewing beneath the
skin
surface. A case of, “well, we were there
anyway so
we cut out some nasty stuff.” It is difficult
to imagine
a surgeon zealously exploring for an unknown
necrosis
in a recently, near fatal snakebite, with all
the added
systemic trauma this entails, without even
the justification
of fasciotomy, but we must conclude that
this is often the case. Contradicting Watt
(1989) who
reports “severe local necrosis” in bushmaster
bite
(probably summarizing Rosenfeld, 1971), I
believe
that surgical debridement is never indicated
under any
circumstances, if that surgery is intended to
relieve a
supposed “venom necrosis.” Even in Bothrops
envenoming,
surgery is probably useful only in managing
infection and gangrene (never to be confused
with
venom necrosis) which usually requires days
to manifest,
and almost always results from too little
antivenom
given at the start, and/or previously
mismanaged
first aid. As Reid (1976) notes (in Russell,
1993):
“By using surgery in all cases … some
necrosis develops
in all ... victims.” In other words, from the
moment the first incision is made the patient
is already
worse off than when he presented.
Watt’s (1989) remark, “Careful, prompt
surgical
management is the key to minimizing damage in
cases
complicated by necrosis” is grossly
underdefined—
just the sort of statement that sends doctors
reaching
immediately for the scalpel. The medical
practitioner
inexperienced with snakebite, confronted with
the rare
case of venom necrosis, believes he is acting
for the
patient’s benefit, and reducing the overall
damage that
would occur. Quite the contrary, excepting
those very
rare instances where surgery has application
(e.g., gangrene),
surgery should never be attempted “promptly”
but only after swelling and inflammation have
receded.
This is a period requiring weeks, not hours
or
days, hence surgery at this time cannot be
considered
“prompt” by any means. In the first days
post-envenoming,
with edema, inflammation and hemorrhage
at its peak, surgical exploration is
diagnostically fruitless:
there will be more damage to come. Presented
with an oozing extremity distorted by
swelling, inflammation
and incoagulable blood, all of which will
have
been aggravated by the surgical incision
itself, few if
any physicians will be able to distinguish
between necrotic
tissue and erythrocytic debris in still
vascular,
living tissue. Yet damage will be increasing
day by
day. Only after the swelling has receded, and
the
destructive agents become static, can the
true extent
of the damage be ascertained. Since local
damage
evolves slowly even if the spread of venom
does not,
it is of little worth to “check the cake
before it is done.”
Because necrosis seems never to start without
hemorrhage,
it follows that the best way to increase
necrosis
is to increase hemorrhage; that is, use
surgery.
And because surgery amplifies the probability
of infection,
and contributes to the shock state by
reducing
the blood pressure, it may even kill the
patient
(see cases below).
Debriding, excising, opening to drain or
clean, or
in any way breaking the skin surface at the
bite site
and surrounding areas increases necrosis and
results
in further degradation of the bitten
extremity. Note
the bite on Judge Carr, in Mole (1924), where
the
fang wounds were lanced and his thumb
withered to
three-quarters normal size; compare to Bites
1-5
(Chapter 22), where the fang wounds were not
tampered
with and no such damage occurred. There
would seem to be no good excuse for using
surgery
in any bushmaster bite, excepting those cases
complicated
by poor treatment methods where infection
had become a greater issue than the
envenoming. In
Bothrops bite, the black, blistered skin at
the fang
punctures and surrounding areas should be
left undisturbed.
This veil of hematose tissue, no matter how
gruesome looking, will desiccate and mummify
as the
weeks progress. Dry and hard and continuous
with
the still venous skin, it will protect better
than any
bandage the compromised underlying tissue.
Hemorrhagic venom necrosis (as opposed to
bacterial
necrosis and other variants) is basically a
kind of scab,
being composed of dead extravasated skin and
dried
hemolytic debris. Cut or tear off this
covering prematurely
and the new tissue beneath it will itself
hemorrhage,
necrotize and/or suppurate, resulting in the
formation of yet another such “veil” of dead
tissue.
Leave the hemorrhagic-necrotic formation
alone, however,
and the dead material, given time, will
slough off
on its own and newly restored skin appear.
Since
sloughing will not occur until well after the
swelling
has receded, and the tissue regenerated (ca.
45 - 90
days), attempts to rush healing with surgery
are not
only pointless but counterproductive. One
must not
yield to the impatience of expecting an
immediate cure
to a condition that is irresolvably chronic
and somewhat
transient, and that requires a long healing
time
before any improvement can be seen; nor
should one
yield to the persuasion of physicians anxious
to “do
something” when doing nothing is the better
course
(bearing in mind that physicians often take
action simply
to satisfy the expectations of the patient).
Viper bite
is not an injury or trauma, it is a disease,
a teleomatic
program evolving, enlarging, changing,
pursuing a
course mosaic, never unidirectional. The
patient
should be informed that he will be
participating in this
“process” which is first not of healing but
of degeneration.
Even with prompt treatment, local damage in
viper bite will generally worsen throughout
the first
week, and if serious, continue advancing for
more
than 20 days. This “program” cannot be
arrested
with a quick-fix like surgery, and cutting
out the damaged
area in an effort to “keep ahead” of the
venom
will only make things a whole lot worse. One
must
begin by protecting the fang punctures and
the eruptions
surrounding them. Every effort must be made
to keep the tissue from breaking so as to
minimize
hemorrhage and exposure to air and bacteria.
It is
precisely where the skin breaks open that
necrosis
and anoxia forms—hence necrosis first appears
within
the fang wounds, bleb formations,
venepunctures, and
other compromised tissue. To preserve the
original
integrity of the bitten extremity should be
the foremost
goal, and frankly, cutting it open is not
much
more sensible than backing your car over it.
I suspect
the results would be much the same in any
event.
The poor overall performance record of
surgery
in snakebite speaks for itself. Russell
(1983) remarks
the general worthlessness of surgery in bites
by North
American crotalids, and Hardy (1992) among
others
have questioned the use of bite excision. A
comparative study of Surgery vis-à-vis No Surgery
in all
snakebite would likely prove my case. Let’s
take a
look at some bushmaster bites in this regard.
Here
the track record of surgery cannot be any
worse—
and can even be linked to the deaths of the
patients.
Hardy and Silva (1998) provide 12 “reliably
authenticated”
envenomings by bushmasters with treatment
details. Add to these the 5 interactive bites
I
described in Chapter 22, and we have a total
of 17
bites where management details known. (I have
omitted
cases of rapid death, and all cases where
treatment
details are not recorded; I have also
included
the case in Mole [1924], where the fang
wounds were
incised.) Here is the score:
Mortalities with surgery 4
Recovery with surgery with lasting
physical disability 4
Recovery with surgery without lasting
physical disability 0
Recovery without surgery and without
disability 9
Even if we acknowledge that the more serious bites that resulted in death and/or
caused disability required surgery to correct the problem, we must admit the
overwhelming failure of surgery to achieve its goals: All deaths involved
surgery, and all cases involving surgery resulted in serious physical
disability. Without surgery, recovery was 100 percent. There is another common
denominator: in all cases ending in death and serious physical disability, all
involved surgery prior to 4 days post-envenoming: the surgery was “prompt.”
Bolaños (1982) reports three fatal cases of bushmaster envenoming with surgery,
and one case of survival with surgery that resulted in physical disability.
“Extensive myonecrosis” was described in all four cases. Note, however, that
myonecrosis prior to surgery could not have been known; it was not a preexisting
complaint of the patients. Indeed, prior to four days (and surgery) there was no
clue to its existence, since a phenomenal lack of skin necrosis was mentioned in
all cases (although in one case some minor necrosis was noted in a small area
around the fang punctures). In effect, the “myonecrosis” was discovered
inadvertently during surgery. Whether this diagnosis was based on confusion with
hemorrhagic cellular debris in the muscle interstices (as in the envenomed mice
in Gutiérrez et al., 1990), or whether it was actual myonecrosis as specified,
is less important than the lamentable outcome of the cases: three of the four
patients died. They did not die of venom necrosis (a condition so rare as to be
unknown), or from the typical hemostatic interruptions of viperine venom. They
died from secondary causes, and on the third and fifth day after the bite. As
summarized by Campbell and Lamar (1989), death resulted from “shock secondary to
massive swelling, suppuration of tissue, and overwhelming infection.
Readers familiar with the snakebite literature cannot fail to note that these
are very strange mortalities. They are even stranger considering the early
antivenom treatment. “Shock, tissue suppuration, and overwhelming infection”
sound more like the effects of septicemia than venom. While too little antivenom
probably laid the groundwork for these deaths (the three patients who died
received only 10 vials each; a fourth patient, who received 20 vials, survived)
no doubt the surgery didn’t do them any good either. Hardy and Silva (1998),
noting from the literature, report that the three patients “appeared to improve
during the initial 36 h, but then went downhill despite continued therapy; the
fourth patient rallied initially and continued to improve.”
What did this “continued therapy” consist of? Obviously surgery (fasciotomy),
during which the “extensive myonecrosis” was encountered and excised. Since
surgery (which requires its own supportive therapy in addition to that of the
snakebite) would more likely be conducted on an improving patient than one in
the death throes (but this only our logic, one that surgeons don’t seem to
have), we may conclude that it occurred before the 36th hour, that is, before
the “improving” patients began to go downhill. Logically, it is likely their
conditions worsened because of their “continued therapy” (surgery) rather than
“in spite” of it. The surgery, occurring prior to 36 h, encouraged the “shock,
tissue suppuration and overwhelming infection” that later killed them. Recall
that all three patients reached medical help early (before 4 h). All received
antivenom and were described as “improving” during the first 35 hours. Yet
something suddenly caused them to go “downhill.” Was it surgery?
There is a fourth bushmaster bite fatality that involved surgery: a case of L.
muta muta bite in Leticia, Colombia. The snake was reported to be over 2.5
meters long (a very large snake). Hardy and Silva (1998) report the victim
received a total of six ampoules of antivenom—two within the first 15 h, and
four thereafter. Since two ampoules within 15 h of a bushmaster bite is little
of nothing (my own severe bite from a much smaller snake required 14 vials, and
was administered within 1 h), antivenom treatment cannot be said to have been
“prompt”. The 4 ampoules subsequently administered (totaling 6) seems even more
inadequate when we consider the snake’s great size and capacity for injecting
multiple lethal doses of venom (Chapters 24 - 25 explores this capacity).
Three days post-envenoming there was evidence of significant infection with
ecchymosis. Coagulation tests were “unremarkable,” which suggests that the
ecchymosis (in the absence of hemorrhagic bullae) with its long delay, might be
due to the intense swelling and infection rather than a hemorrhagic effect of
the venom. On the third or fourth day post-bite, the extremity was subjected to
“extensive surgical debridement through an anteromedial incision of the lower
leg, and extensive hemorrhagic necrosis of the muscle was encountered.” The
patient died within 24 hours of the surgery, from “irreversible
hypotension.”
Perhaps we have stumbled upon a formula for insuring that bushmaster bite lives
up to its reputation and kills the patient regardless of our efforts to save
him. This formula consists of two simple ingredients: too little antivenom and a
lot of surgery—surgery to remove a muscle necrosis that the surgeon cannot be
sure is there until he has operated (during fasciotomy), and perhaps cannot even
properly identify once he has; but that is, at any rate, much less dangerous to
the patient’s life than the surgery that proposes to correct it. Within the
melange of inflamed and nearly unrecognizable tissue encountered once breaking
the edematous surface of the skin, the view obstructed by hemorrhagic debris,
probably only subsequent putrefaction could make “necrosis” apparent to the
surgeon. And such “necrosis” would as likely result from the additional damage
of the surgery (from anoxia) as from any verifiable effect of the venom. No
matter, even here surgery should fail its task, since in these early days the
advancing process of the envenoming (for snakebite, as I say, is not an injury,
but many, many cumulative injuries evolving along a chemical time-chain) should
continue long past the initial incision.
In the four cases in Bolaños (1982), extensive myonecrosis with no skin necrosis
is a strange thing. Skin necrosis was seen in only one patient, confined to a
small area around the fang punctures. The long fangs had evidently injected the
venom so deeply into the muscle as to have bypassed the skin. Since bushmaster
fangs may reach 3 cm (and penetrate to a depth of 4 - 5 cm with the compression
of the bite) this is not impossible. Yet in my four bushmaster envenomings, and
in the bite on the herpetoculturist in New York State, there was no necrosis of
any kind, not even at the fang wounds. With the shock effects that surgical
intervention may only extend or complicate, we can see that necrosis is the
least of the patient’s worries. Even if muscle necrosis were a reliable (and not
misidentified) occurrence, surgery to correct it is at best inappropriate during
the early days post-envenoming, and should not be performed until the patient
has made a full general recovery. Venom necrosis is not life-threatening—surgery
is! Venom necrosis is not bacterial necrosis, which is of a distinct character.
The lethal action of bushmaster venom is primarily an effect on blood
distribution, and any restorative effort should first concentrate on managing
these much more dangerous shock effects, even to ignoring local damage, no
matter how dramatic or apparently severe. At no time should surgery be performed
on the extremity until the patient is well past the danger zone—when, in other
words, systemic alterations have entirely abated. Surgery advances the
hypotensive state and thus precipitates total cardiovascular failure. The
physician should be persuaded to note that only after the edema and inflammation
has receded (requiring sometimes 6 weeks or even more) can a final appraisal of
the local damage be made, and that surgery prior to this time is not only
premature, it will aggravate the problem.
To date I have been envenomed by 11 viperid species.* These include: Atractaspis
(with necrosis), Causus, Porthidium, Bothrops asper (with necrosis), B.
leucurus, Bothriechis schlegelii, an immense Agkistrodon piscivorus (when I was
a 90 lb, 13-year-old boy; this required 14 days in ICU and a year’s therapy to
regain use of my right hand). I have had four bites by Lachesis species, two in
the severe category [Ed. note: Ripa’s fifth, sixth, and seventh bushmaster bite
predates this text]. All these involved intense pain, inflammation, pronounced
and in some cases massive swelling, various degrees of tissue destruction and
deficits of mobility resolved only after a very long recovery time. All the
bites occurred on my hands or digits. The reader will be heartened to learn,
however, that I am typing this manuscript with all ten fingers! Had “prompt
surgical management” been performed in each of my cases, I wonder how many
fingers I would have left? Indeed, I should by now resemble a maimed circus
freak with flapping noodles for arms and living off disability. And yet I have
no discernible scars, save one resulting from the clinical lancing of the fang
punctures (in the Agkistrodon bite), a relic of the old days when “cut and suck”
was still practiced even in hospitals. The other ten bites, despite necrosis in
some of them, healed without scarring. Thus, the only scar I have sustained out
of 11 viper bites involved the scalpel!
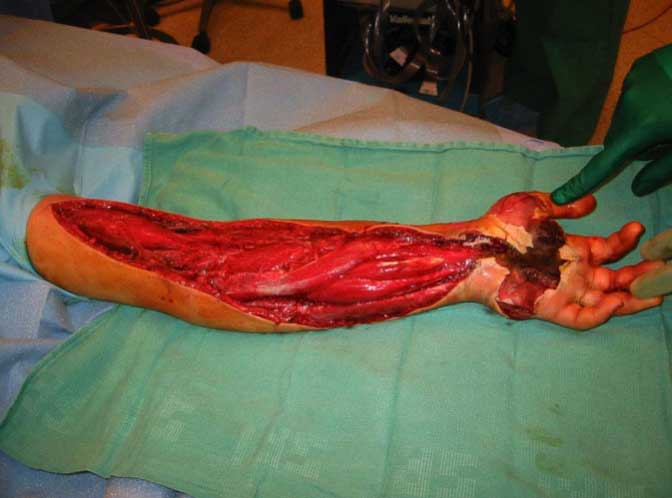
Figure 5 (above). Insane futily fueled by the medical wive’s
tale of “compartment syndrome.” Rattlesnake bite on 13-
year-old male treated with fasciotomy.
The literature is a reservoir of vague,
unfounded,
and misleading diagnoses for under-defined
symptoms,
crudely drawn against a background of often
arbitrarily proposed terminologies. Necrosis,
that all-
purpose term for any condition where tissue
is irrevocably
damaged has been blamed more on venom
when it should have been blamed more often on
bacteria,
iatrogenia, and anoxia from surgery. In
Figures
17 - 20, I reclassify necrosis according to
its causes
and symptoms, and suggest that different
types of
necrosis require different kinds of
management.
Another factor commonly misevaluated is the
permanency
of symptoms. Dart et al. (1992) arbitrarily
defines as “permanent” any alterations
persisting for
more than one month. Would that venom
finished up
with us so quickly! At one month the limb may
still be
“in the cooker,” as it were, with symptoms
still escalating,
while in other envenomings the damage will
only
be starting to recede. Snakebite is not an
injury, it is
a disease. It is a process resulting from an
introduced
chemistry that, like the cancer whose
molecular structure
venom more than discretely resembles,
advances
through stages. These stages cannot be
interrupted
by surgery! Only living tissue transmits
venom to other tissue! As in cancer,
envenomation
is a program in which the victim’s cellular
structure
and mode of chemical exchange participate in
the cell’s
own breakdown. Indeed, there are forms of
necrosis
where the cells so react to the actions of
the venom
as to mimic it, auto-destroying the tissue
and even
killing the patient! And this even though the
actual
venom has been neutralized! This Delayed
Hypersensitivity
Necrosis (DHN; Figure 17-20) is inspired
by anoxia from surgery and is the only kind
of venom-
induced (non-bacterial) necrosis that can be
described
as systemic and fatal.
We must be very careful when we speak of
permanency
in snakebite. Granted this terminology may
be only a methodological convenience for
classifying
some symptoms in a text (e.g., as in Dart et
al., ibid.),
it can only create confusion on the
battlefront where
use of invasive means hinges on the
diagnostic talents
of the physician who may thus construe damage
lasting
longer than one month to be literally
permanent
and so advise surgery accordingly. In fact,
one can
expect local alterations in any serious viper
envenoming
to last for upwards of one to three months as
a
matter of course. Some deficits may last
greater than
a year in many cases. Hence, after six weeks
when
the limb is still livid and swollen and
hemorrhagic necrosis
has not yet spontaneously resolved (but might
if given more time), some physicians might
advise invasive
means to correct this seemingly “permanent”
problem. This can only result negatively for
the patient,
who should be patient a little longer, please
—
lest he wish his condition to be made to fit
the Dart et
al. (1992) definition forever. Contracture,
joint stiffness, hyperplasia, loss of sensitivity, &c.,
can be expected
to last many months, but these conditions
stand
a better chance of resolving on their own
than with
surgery.
Perhaps the danger with the advice given in
Watt
(1989) and others lies in the vaguely defined
terms.
“Prompt surgical management” and “complicated
by
necrosis” are just malleable enough
statements as to
be without practical meaning. What exactly
are the
complications of necrosis and doesn’t surgery
itself
promote many of them? Doctors naively
following
Watt’s (1989) advice will have no idea what
“prompt”
means in regard to necrosis and begin
debriding tissue
as soon as it appears. By this process well-
intended
surgeons, through a hideous progression of
operations resembling whittling, convert
healthy arms
and legs into crippled, useless nubs—what I
call the
“death by a thousand cuts” method. Each week
a
smiling executioner shows up at your beside
and
carves off a little more of you—renewing your
necrosis
into the bargain, at no extra charge! The
photography
in this chapter discloses some pretty graphic
examples.
Hemorrhagic necrosis does not harbor or
retain
venom—and being dead and non-vascular it
cannot
further transmit venom to the underlying
tissue. It is
not literally “rotting flesh” and does not of
itself constitute
a source of bacterial infection. To remove
this
hard, desiccated veil of protective tissue is
to invite
infection into the wound, increasing tissue
anoxia and
perhaps even enkindling the dreaded
catastrophic necrosis
(DHN), by which model we observe certain
spider venoms (e.g., Loxosceles sp) can
devour (de-
flesh) an entire human body over a period of
days.
And yet here it is not the venom but the body
that is
eating itself! The venom is only a trigger-
mechanism.
At least some forms of necrosis are
imitative, born of
disturbed cellular program-sharing. The cells
replace
themselves with unfit counterfeits engineered
for an
early death. Here, the similarity of venom to
cancer
becomes obvious. Venom is deadly but it is
also information.
It takes “two” to make a poison, and it is
the victim who translates the codes.
“Complicated by necrosis” elicits only the
vaguest
judgment call—what seems to be implied is
that the
necrosis itself is the “complication.” Does
the writer
mean complicated by infection? Then treat the
infection.
Does he mean complicated by gangrene?
Gangrene
and venom necrosis are two completely
different
conditions and should be treated as such. Gangrene spreads, having an origin not
in venom
but in
bacteria. Venom necrosis becomes rapidly
inert—the
venom that caused it will have already
infiltrated the
tissue well before the physician sees the
case. Its activity
is short, usually about 3 - 5 days (if not
surgically
tampered with), and by 17 - 20 days will be
in
remission. If the necrosis persists past this
period it is
not venom necrosis; it is either imitative
(programmed
by an altered chemical exchange from
surrounding
cells), or anoxia stemming from secondary
causes.
My review of different types of necrosis
(Figures 17
-20) shows just how complex the presentation
can
be. Surgical management, if it is used at
all, should
proceed cautiously toward specific
etiologies, and in
writings on the subject, physicians should
not be left
to define these terms haphazardly, for
themselves. A
clear cut guide needs to be developed. In
cases where
days have elapsed before the patient has
sought medical
help, where antivenom has not been used (or
after
its use is no longer efficacious), or when
poor first
aid measures (such as tourniquets or
cryotherapy) have
been employed resulting in damage secondary
to the
venom, perhaps here and only here can
invasive methods
be indicated in snakebite—albeit as a last-
ditch
action. But the working physician, who may
never
have seen a snakebite before, will not have
the least
clue what “prompt surgical management” means
when
presented with a massively swollen extremity
bubbling
with bullae.
The recent case of an agricultural worker and
part
time snake hunter in Costa Rica, Miguel X, is
a prime
example of what not to do in a snakebite.
Bitten by
an adult bushmaster on the forearm, and
although receiving
prompt antivenom treatment (200 ml), Miguel
had the misfortune to meet a good surgeon
before
escaping from the hospital. A fasciotomy was
promptly
performed, and thereafter some necrotic
tissue was
removed each day for one week from the muscle
(pers. comm, A. Solórzano). Note, however,
there
was no skin necrosis in this case—all
necrosis occurred
in the clinically altered underlying fascia
and
muscle. Note also that even after the initial
necrosis
was removed, debridement continued on a daily
basis
as new necrosis developed. Not surprising in
a
gaping 9 x 16 cm crater cut to sub-facial
depth, exposing
muscle, tendon and bone during the early
healing
process! Here is a clear-cut case of necrosis
amplified
by surgery, enhancing anoxia and encouraging
hemorrhage, additionally exposing the
affected tissue
to oxygen and bacteria. A year’s investment
in split-
skin grafts has not restored Miguel’s arm to
normal
appearances, nor is it likely that it will
ever regain
normal function. More tragic is a recent case
in southeastern
Peru (recorded in Mellor and Arvin, 1996)
where early surgical tampering in what was
probably
not even a severe bite (my view, not the
authors), led
to the amputation of a man’s leg at the hip.
Thousands
of such mismanaged cases occur every year in
Latin America, Asia, Africa, and even the
United
States—victims of “prompt surgical
management.”
One doctor in Suriname told me he routinely
performed
fasciotomy in every case of snakebite,
regardless
of the severity, and this usually entailed
excision
of the bite area as well! How many
mutilations
had this one man performed in his lifetime on
guileless
patients who might have been better off
trusting the
local witchdoctor? Perhaps there is more
sound advice
to be had from America’s religious snake-
handlers
(who endure venomous snakebites on a regular
basis and most without serious disability)
than from
physicians who, in this modern age, still
practice such
witchcraft routinely. A survey conducted on
the entire
five-state membership of the Pentecostal
church
might find less maimed individuals
comparatively—
people who scorn all hospital treatment,
including
antivenom. Ultimately, the responsibility
must rest with
those medical authors who persist in making
claims
for the success of surgery in spite of
mounting evidence
to the contrary, or who use hastily concocted
or vague terminologies that provide no clear
diagnostics
for continuing this outmoded, damaging, and
dangerous
procedure.
The type of necrosis determines the type of
treatment
This is a matter ignored by most if not all
writers on
snakebite. Yet its importance cannot be too
strongly
emphasized. Venom triggers various responses
ending
in necrosis, and different kinds of necrosis
can be
observed. In general, necrosis results from:
(1) The primary necrotic agents of the
venom. Rare
(2) Hemorrhagic effects of the venom
(recognizable
by erythrocytic debris; this will appear
blackish
and hard). Common
(3) Deficits in blood circulation (e.g.,
vasoconstriction),
and this may be combined with either of the
above conditions. Rare.
�
(4) Tissue anoxia due to deficits of blood
circulation
caused invasive (e.g., surgical) or
mechanical
means; i.e., iatrogenic treatments
(tourniquet,etc.).
Common.
(5) Secondary infection. Common.
(6)Autoimmune reaction (delayed type
hypersensitivity).
Rare.
In severe envenomings by vipers probably some
or even all of the above effects will be
seen, although
they should be minimized with prompt
immunotherapy.
Corrective surgery should be used only as a
last
resort, however. Hemorrhagic damage presents
as
a hard, fibrous scab and this material,
though quite
dead, should be left in place. It acts as a
barrier to
expanding necrosis and secondary infection.
Necrosis
has a tendency to follow behind surgery, thus
each
time more tissue is debrided more necrosis
appears.
By slow increments the surgeon’s knife creeps
up the
limb—the “death by a thousand cuts” method of
incremental
amputation. Hemorrhagic necrosis, by far
the most common form, increases with a
weakened
cellular wall, hence it will always bloom
first at the site
of an incision. A good way to give your
patient a
serious or even fatal infection is to
promptly excise
the inoculation area (or other tissues
damaged by venom),
eliminating a natural protective barrier to
bacteria
and weakening the tissue wall against further
venom
hemorrhage. If the patient has already
presented
with an infection, then it is likely that
antivenom has
not been given in time or has been given in
low quantity,
and surgery to deal with sepsis may be a
matter
of course. However, true venom necrosis is
best dealt
with non-invasively. Certain serious
infections (such
as gas gangrene) will probably require some
invasive
management regardless, realizing that too
early debridement
of envenomed tissue where it is not warranted
may be laying the groundwork for a later
infection
that would not otherwise have occurred. An
autoimmune reaction resulting in catastrophic
necrosis
gradually overtaking the entire extremity
(resembling
a heparin-induced thrombocytopenia) is
usually
secondary to invasive wound management. As
this
will always be complicated by infection, it
is very difficult
to differentiate. Delayed Type
Hypersensitivity
Reaction (DTHR) is characterized by swelling,
redness,
an influx of macrophages and the production
of
tumor necrosis factor (TNF) and interferon-g
(IFNg).
This type of necrosis, resulting in a
“spontaneous
necrotizing fascitis,” is diagnostically a
false-positive,
blamed on envenomation, but the later may be
only one factor triggering the effect. Surgery
enhances this
condition rather than relieving it.
Is fasciotomy for you?
It is not in a snake’s best interest to cause
edema
in the prey animal. A rodent too swollen to
move
might also be impossible to swallow!
Moreover, if
edema served a digestive function it would
not
“work”—the prey would be dead before the
swelling
could take place. From this it can be deduced
that
venom did not evolve agents especially to
cause edema;
there is no reason for natural selection to
retain
these chemicals in the venomous repertoire.
Edema,
rather, is the victim’s contribution, a
response to toxins
evolved for other purposes, developing only
in
those larger non-prey animals (like man) that
survive
long enough to exhibit this symptom.
The problem with edema is contextual; an
“abnormality”
appears and the physician tries to correct
it. He fails to see that edema, in fact, is
the most
normal part of the envenomation and that if
he did not
see edema he should be observing a true
abnormality—
a sign, perhaps, of something even more
gravely
wrong with his patient’s immune response. We
should
not think of venom as “causing” edema;
rather, we
should recognize that it is the body’s own
contribution
to the envenoming. The body is reacting to
the
venom autopharmacologically, with edema as a
protective
strategy. Gross swelling is a purposeful
mechanism
evolved to dilute the mass of the venom with
a
yet greater mass, past the point where the
venom can
do fatal damage to the organism. Swelling
performs
a biologically useful role as a defense
against tissue
damage, serving to expand the cellular wall
with sheer
water mass and prevent concentration of the
destructive
substance at the envenomation site, as well
as
block its communication past these fluid
barriers.
Decrease the swelling prematurely and you
will inadvertently
increase this destructive concentration
taking
place. There is not a single verifiable case of
edema alone
contributing to loss of limb in snakebite. On
the contrary,
the cases of mechanical means to decrease
swelling (e.g., ice-water, fasciotomy, etc.)
contributing
to loss of limb are too numerous to recount.
The term “compartment syndrome” (the
allegedly
dangerous symptom which fasciotomy allegedly
relieves) is so grossly under-defined as to
have no therapeutic relationship with real-life
situations and no
meaning outside of a medical dictionary.
Intended to
describe a condition where edema becomes so
intense
as to compromise vascularization and
constrict
nerve tissue, it has instead become a
clinical catchall
for any severe swelling “causing pain on
passive
stretch, hypesthesia, tenseness of
compartment and
weakness.” Since all these symptoms are
concomitant
in snakebite, the diagnosis is muddled from
the
onset. Compartment syndrome is a word game,
and
the methods used to test for it evolved from
a mythical
preconception about a never documented
result,
responding in a knee-jerk way with a never
well comprehended
traditional approach. “Compartment syndrome”
might better be named the we-don’t-
knowsyndrome.
When the surgeon responds with fasciotomy,
it is simply because he observes a lot of
swelling,
thinks it’s “bad” and “doesn’t know” what
else to
do about it. Not a very safe proposition for
the patient.
Medicine has a long history of iatrogenia and
a lot
of what has come down to us as “modern
therapeutics”
are only reactions against the ill-fated
treatment
methods of the past. Probably the idea of
“compartment
syndrome” in snakebite arose as a reaction to
the widespread use of tourniquet
constriction, and is
a relic from the days when tourniquets were
freely
used even by doctors. What doctors blamed on
swelling they might better have blamed on
their own
faulty treatments. To date edema has never
yet been
proven to result in any permanent damage that
could
not otherwise be attributed to the cytotoxic
effects of
the venom itself. Physicians still defend the
use of
fasciotomy on the basis of an apparent but
never directly
proven effect. Fasciotomy always results in
greater deficit to the afflicted extremity
than would
otherwise have occurred without it.
Advice to physicians: snakebite + surgery =
infection
(bacterial necrosis), tissue anoxia, delayed
type hypersensitivity response (catastrophic
necrosis),
amputation, shock, death and/or the absolute
certainty of some disfigurement and deficit.
Non-invasive medical management of snakebite
(e.g.,
with drug therapy and other nonsurgical
methods)
offers increased chance of full recovery with
no long
term physical deficit or disfigurement.
Fasciotomy has no value in preventing or
controlling
necrosis (Russell, 1983). Its efficacy has
never
been proven (Dart, 1999). Its success has
been justified
by a false positive, justified by an unknown
outcome. Performed primarily as a prophylactic
measure,
it persists because no evidence can ever be
salvaged
to show what might have happened had the
procedure not been performed. The logic for
fasciotomy
is a logic by default. It endeavors to save
the
limb by correcting an averred “abnormality,”
and ends
up losing the limb and often the patient into
the bargain.
It adds trauma to an already traumatic
situation
and increases mortality through increasing
hypovolemic
shock. It is a political exercise as much as
a medical
one. It is used because it satisfies the
patient’s expectations
of the physician to produce concrete action
in
the face of massive swelling and the
physician’s need
to satisfy his own legal liability.
Perhaps the use of fasciotomy in the modern
day
has more to do with malpractice insurance
than science.
It persists because physicians can be held
accountable
for treatments withheld (e.g., “the doctor
has not done everything in his power”), and
are held
less in account for treatments given (e.g.,
“the doctor
has done everything in his power”). By
performing
fasciotomy, he will be protected by the
complexity of
sequelae in an outcome that can never be
positively
determined against him. Consciously or
unconscious-
ly—he may fully believe in the efficacy of
his actions—
he acts less for the patient’s behalf than
for his own.
Thus, fasciotomy, without any clear evidence
to support
its use, persists in the medical literature
as a viable
treatment for snakebite. This butchery is
widespread,
being performed in almost every country in
the world and in some regions as routinely as
the use
of antivenom itself! “Better safe than sorry”
is the
tag-line justifying it. Tragically, one is
even more unsafe
and far more sorry the moment Mister Surgeon
enters the treatment room.
So long as fasciotomy is permitted in any
cases, it
will be used in all cases where swelling is
severe.
And swelling is always severe in genuine
envenomings
by viperid snakes and many elapids as well.
Fasciotomy is routinely more damaging than
the purported
“compartment syndrome” it proposes to
relieve.
Fasciotomy always causes some deficit,
whereas
“compartment syndrome,” as so vaguely
defined,
has never yet been shown to cause any deficit
in snakebite.
Deficit caused by fasciotomy is a
mathematical
certainty. Deficit caused by a “compartment
syndrome”
is an unknown, a remote possibility at best.
Hedging one’s bets against a mathematical
certainty
in favor of an unknown is bad medicine.
�
Fasciotomy may safely be put to rest along
with
the cruciform techniques of lancing and
sucking bite
wounds that have also persisted from prior
centuries
as a treatment for snakebite. Surgical
debridement
should never be conducted except to relieve
infection
(but only if that infection cannot be
controlled by noninvasive
means) and should not be performed solely
to correct hemorrhagic venom necrosis.
I have endured edema so tense that even to
twitch
the skin was to cause it to split open; and
yet I for all
that, I would never even consider going under
the
knife in a snakebite. If I were asked when
and by
what diagnostics I would accept fasciotomy to
treat
one of my own envenomings, my reply would be
simply
this: only when I can no longer feel pain or
touch
in the extremity, when the limb has lost all
response to
neural responses and commands—when, in short,
it
has gone completely numb. I have never
experienced
this symptom, and I do not know anybody else
who
has either.
The origin of snakebite treatment:
therapeuticexorcism?
Alien anthropologists landing for the first
time in our
frightened little world, and having no
inherited fear of
snakes as we do, might conclude that use of
certain
treatments in snakebite reflects a deeper
cultural origin
than that of a well-intended science.
Crosscutting
throughout human history, they might link our
curative
practices not to any provable success rate,
but to a
religious esoteric older than medicine
itself. Certainly
all diagnoses, and the actions taken, spring
from the
neurological (psychological, semantic,
cultural, etc.)
dispositions of the actors first, before they
find their
way into the medical room. We comply with
certain
traditional practices not because they are
proved or
provable, but because belief-inertia makes us
incapable
of resisting them. We take pills not because
we
need pills (although we may need them); we
take them
because we believe in them and expect to be
given
them, and to give them in turn. The
correlation with
pills and cures can range from to zero to any
figure
you can imagine; but the correlation with our
desire
to both give pills and receive pills is 100
percent. The
psychological need for some form of treatment
will
always dominate its curative effect.
We live in a world of false positives,
medically
prescribed and scientifically “proven.” Our
success
rate is higher than in ancient days, but a
wild randomness
has guided us here, through a series of magic
tricks that work—sometimes—and sometimes do
not.
When they work, our magic is “good”; when
they do
not work, the “evil humors” were too strong.
The
healer’s art arose from shamanism, not
Merck’s handbook,
an art evolved from effects that seem magical
to the patient, and hardly less so to its
modern inheritors
and practitioners, proudly parrotting the
spells
and incantations of other medicine-men before
them.
Over the ages, powerful correlations with
chance have
bequeathed our book-learned shaman a
reductionist
philosophy called “science” from which to
draw (and
exhibit) power, but the lots are still cast
in the sand,
and the entrails read, though they be our own
entrails
sometimes, explored for misguided cells
rather than
for misguided demons who do not belong there.
Medicine arose from just such a wild
randomity, a
psychological slight of hand to make us
forget our
desperation when confronted with forces we
could
not overpower—and letting the witch doctor
taking
credit for our immune systems. In this game
“he who
rattles the bones loudest, wins.” Viewing
snakebite
treatment chiefly as the artistic expression
of its practitioners,
and secondly, from the psychological needs
of the victim to receive a particular kind of
treatment
that favors his cultural/religious
expectations, we find
the doctor-patient relationship exists as a
sort of devil’s
bargain where two residual forces work hand
in
hand in the battle against a superstitious
evil both doctor
and patient commonly believe in. So long as
both
actors believe in the same devil, you have a
sound
business deal. The medical artist fulfills
the expectations
of the patient—he cannot stray very far and
still
have a happy customer. If it is better to do
nothing at
all, the doctor must yet do something because
that is
why the patient is there, to see something
done. Snakebite
treatment, which has not advanced
significantly
since the development of antivenom more than
a hundred
years ago, has developed no acceptable new-
age placebo by which to work its special
effects. We
are past the stage where eating certain
leaves or doing
a certain dance will be believed in by the
patient.
Mere antivenom has ceased being exciting to
the fast-
talking interactions of modern technological
salesmanship.
More complex formulas, the more elaborate
the better, win the day, and win the
patient’s confidence.
This has happened in all forms of medicine,
which has become so technologically elitist—
e.g., “ah,
but we have the latest laser!”—that the
Hippocratic
Oath has been thrown out with the patient.
The greatness
of medical progress has been to make itself
un
�
affordable to nearly everyone in America, a
glorious
state that the corporate money-powers, with
their guns
to the heads of our politicians, mean to
export to the
rest of the world as well. The history of
snakebite
treatment follows just such a dependency: a
competitive
technology that so early-on exceeded its own
abilities to do anything new that it reaches
back frequently
into the ritual smoke it sprang from, out of
sheer desperation to keep up with style. A
treatment
that ought, at most, to cost a few hundred
dollars in
antivenom and fluids, now costs thousands of
dollars
in mind-boggling blood testing (to reassure
us of what
we already know, that there are clotting
problems),
unnecessary surgery, and all the rest, just
to show us
that our doctors are using the latest and the
best. So
snakebite treatment plods on, looking for
something
new to do, or be. One year you have an
electric
stun-gun, the next you have an “extractor”;
even the
antivenom is being monkeyed with, requiring
gallons
of it nowadays (e.g., CroFab) whereas a few
vials
worked just fine formerly. Rife with
ritualized expressions,
relics from the witch’s circle and the
medicine
tent, snakebite treatment continues to
mystify
both patient and practitioner alike, while
physicians
blindly ransack a grab-bag of never very
successful
materials and methods in the hope of keeping
up with
Doctor Jones. As of this moment, somewhere in
the
world, somebody’s foot or hand is being split
open,
cauterized, branded, frozen, strangled,
slashed, rubbed
with painful crystals, excised, electrocuted,
or amputated
straight away. One-sided affairs in which I
am
afraid the doctor is having all the fun,
promoting an
idea more religious than curative.
Why do the Judeo-Christian countries (where
snakes are equated with evil) lean toward
violent,
aggressive treatment of snakebite, rather
than toward
the more passive approaches taken in tropical
animistic
societies where snakes hold more of a
regenerative
role in mythology, rather than an
antagonistic
one? Why does surgery appeal to the Western
mind
as a better alternative than say, eating
special leaves
and drawing poultices? Certainly the cure
rate is not
greater when antivenom is not used, and
snakes are
even more venomous in the tropics. Released
from
its apparent intent (which is to cure), what
does the
artistic expression of surgery (of all
possible forms of
treatment selected) represent to both the
practitioner
and his patient (who must give ultimate
approval for
its use)? Is it a subconscious need of the
physicians
to excise (read exorcise) the evil of the
snake, abetted
by the patient whose expectation is to see
the evil
excised? Does the method of treatment reflect
the
moral expectations of our society, an
acceptable means
of retribution against the serpent “whose
evil spirit yet
lives within the wound?” A recidivistic case
of, If thy
right hand offends thee, cut it off—? The
patient
contributes through his own tacit
expectations, perhaps
needing to be punished for his congress with
the
demon-snake (his blood diluted by the devil’s
substance
becomes spiritualized, a sin) with only the
most
radical and violent ritual capable of
expiating him. The
cruciform brand of the old “cut and suck”
method
evolved from a more invocative than practical
strategy;
the carving of a sacred cross over the
devil’s
marks, to drive the demon out.
The Pentecostal snake-handlers do not
require human
intercession to banish their devils—they have
a
patriarchal God who asks only faith for His
fee. But
the scientific heathen abandoned in the
techno-wilderness,
must extract his cures from an increasingly
material realm. Divorced from “divine
contact,” and
urged on by vague impulses no less beyond his
understanding
than those of his less enlightened
forbearers,
he digs frantically with his knife in order
to banish
the mysterious force of nature whose
pharmacology
both intrigues and horrifies him. His Gods
arise and
appear not in religious tracts, but in the
equally dogmatic
assertions of other scientists. Were our
extraterrestrial
visitors Freudians as well as aliens they
might
diagnose other causes, such as those
originating in
childhood; a puerile curiosity to see what is
inside so
gruesome an item as a snake-bitten hand or
foot,
which, swelling up with fluid, becomes
phallic; the gratification
of taking complete license with the body of
another person, of splitting end to end the
monstrously
swollen member and watching its insides
avulse—a
deeply personal activity between consenting
parties,
medically justified. Old demons die hard.
�
Literature Cited and Related Reading
Beers, M. H., and R. Berkow. 1999. The Merck
manual
of diagnosis and therapy. Merck Research
Laboratories.
Whitehouse Station, N.J.
Bjarnason, J. B., and J. W. Fox. 1994.
Hemorrhagic
metalloproteinases from snake venoms.
Pharmacol Ther
62, 325-372.
Blaylock, R.S. 2000. Antibacterial properties
of KwaZulu
Natal snake venom. Toxicon. 38:1529-34.
Bogert, C. M. 1943. Dentitional phenomena in
cobras
and other elapids, with notes on adaptive
modifications.
Bull. Am. Mus. Nat. Hist. 81: 285-360.
Bolaños, R. 1972. Toxicity of Costa Rican
snake venoms
for the white mouse. Amer. Jour. Trop. Med.
Hyg. 21:360
363.
Bolaños, R. 1982. Las serpientes venenosas de
Centroamérica y el problema del ofidismo.
Primera parte.
Aspectos zoológicos, epidemiológicos y
biomédicos. Rev.
Costarr. Cienc. Méd. 3:165-184.
Bolaños, R., G. Muñoz and L. Cerdas. 1978.
Toxicidad,
neutralización e immunoelectroforesis de los
venenos de
Lachesis muta de Costa Rica y Colombia.
Toxicon
16:295-300.
Bolaños, R., O. Rojas and C. E. Ulloa Flores.
1982.
Aspectos biomédicos de cuatro casos de
mordedura de
serpiente por Lachesis muta (Ophidia:
Viperidae) en Costa
Rica. Rev. Biol. Trop. 30:53-58.
Campbell, J. A. and E. D. Brodie, Jr. (eds.)
1992. Biology
of the pitvipers. Tyler, Tex: Selva.
Campbell, J. A. and W. W. Lamar. 1989. The
venomous
reptiles of Latin America. Ithaca, NY:
Cornell University
Press.
Cedergren, E, Johansson, B., Heilbronn, E.,
and Widlund,
L. 1973. Ultrastructural analysis of
phospholipase A
induced changes in membranes of synaptic
regions in
rat motor cortex. Ex. Brain Res., 16:400.
Damico, D. C., S. Lilla, G. de Nucci, L. A.
Ponce-Soto,
F. V. Winck, J. C. Novello, and S. Marangoni.
(2005).
Biochemical and enzymatic characterization of
two basic
Asp49 phospholipase A2 isoforms from Lachesis
muta
muta (Surucucu) venom. Biochim Biophys Acta
1726,
75-86.
Dart, R. C., J. T. McNally, D. W. Spaite and
R. Gustafson.
1992. The sequellae of pitviper poisoning in
the United
States. In: Biology of the Pitvipers, (eds.)
J. A. Campbell
and E.D. Brodie Jr. Tyler, Tex.: Selva.
Dart, R. 1999. Int J Med Toxicol 1999;
2(1):1, Hall EL.
Ann Emerg Med 2001;37:175-80).
DaSilva, N. J., S. D. Aird, C. Seebart and I.
I. Kaiser.
1989. A gyroxin analog from the venom of the
bushmaster
(Lachesis muta muta). Toxicon 27:763-771.
Diniz, M. R., and E. B. Oliveira. (1992).
Purification and
properties of a kininogenin from the venom of
Lachesis
muta (bushmaster). Toxicon 30, 247-258.
Ditmars, R. L. 1910. Reptiles of the world:
Tortoises
and turtles, crocodilians, lizards and snakes
of the eastern
and western hemispheres. NY: Sturgis and
Walton Co.
Esquerre, C. 1976. The minimum lethal dose
(MLD) of
venoms from Peruvian Crotalidae Snakes. San
José, Costa
Rica: Fifth international symposium on
animal, plant, and
microbial toxins abstracts.
Estep, K., T. Poole, C. W. Radcliffe, B.
O’Connell and
D. Chiszar. 1981. Distance traveled by mice
(Mus
musculus) after envenomation by prairie
rattlesnakes
(Crotalus viridis). Bull. Psychon. Soc.
18:108-110.
Estevao-Costa, M. I., Diniz, C. R.,
Magalhaes, A.,
Markland, F. S., and Sanchez, E. F. 2000.
Action of
metalloproteinases mutalysin I and II on
several
components of the hemostatic and fibrinolytic
systems.
Thromb Res 99, 363-376.
Fan, H. W., and J. L. Cardoso. 1995. Clinical
toxicology
of snake bites in South America. In: J. Meier
and J. White,
(eds.) Handbook of clinical toxicology and
animal venoms
and poisons. New York: CRC press.
Faraci, F. M., M. A. Klotz, H. W. Shirer, A.
Orr and J.
W. Trank. 1980. Characteristics of bulbus
cordis
mechanoreceptors in the pond turtle Pseudemys
scripta
elegans. Trans. Kansas Academy of Sciences
83(1):132
1980.
Felicori, L. F., C. T. Souza, D. T. Velarde,
A. Magalhaes,
A. P.Almeida, S. Figueiredo, M. Richardson,
C. R. Diniz,
and E. F. Sanchez. 2003. Kallikrein-like
proteinase from
bushmaster snake venom. Protein Expr Purif
30; 32-42.
�
Feres, T., E. Frediani-Neto, TB Paiva. 1994.
Mechanism
of smooth muscle contraction and relaxation
mediated
by kinin receptor. Braz J Med Biol Res
27:1911-6.
Fitch, H. S. 1981. Sexual size differences in
reptiles.
Miscellaneous Publications of the Museum of
Natural
History, University of Kansas 70:1-72.
Fuly A.L., S. C. Elias, R.B. Zingali, J. A.
Guimarães, and
P. A. Melo. Myotoxic activity of an acid
phospholipase
A2 isolated from Lachesis muta (Bushmaster)
snake
venom Toxicon 2000; 38: 961–72
Gutiérrez, J. M. 1995. Clinical toxicology of
snakebite
in Central America. In: J. Meier and J.
White, (eds.),
Handbook of clinical toxicology of animal
venoms and
poisons. New York: CRC Press.
Gutiérrez, J. M. and F. Chaves. 1980. Efectos
proteolítico, hemorrágico, y mionecrótico de
los venenos
de serpientes costarricenses de los géneros
Bothrops,
Crotalus y Lachesis. Toxicon 18:315-321.
Gutiérrez, J. M., F. Chaves and R. Bolaños.
1980. Estudio
comparativo de venenos de ejemplares recién
nacidos y
adultos de Bothrops asper. Rev. Biol. Trop.
28:341-351.
Gutiérrez, J. M., F. Chaves and R. Bolaños.
1990.
Ontogenetic changes in the venom of the snake
Lachesis
muta stenophrys (bushmaster) from Costa Rica.
Toxicon
28:419-426.
Gutiérrez, J. M., G. Rojas and L. Cerdas.
1987. Ability
of a polyvalent antivenom to neutralize the
venom of
Lachesis muta melanocephala, a new Costa
Rican
subspecies of the bushmaster. Toxicon 25:713-
720.
Giovanni-De-Simone, S., A. S. Aguiar, A. R.
Gimenez,
K. Novellino and R. S. de Moura. 1997.
Purification,
properties and N-terminal amino acid sequence
of
kallikrein-like enzymes from the venom of
Lachesis muta
rhombeata. J. Protein Chem. 16(8):809-818.
Hardy, D. L., Sr. 1992. A review of first aid
measures
for pitviper bite in North America, with an
appraisal of
the Extractor (TM) and stun gun electroshock.
In: J. A.
Campbell and E. D. Brodie Jr. (eds.), Biology
of the
pitvipers. 405 - 14 Tyler. Tex.: Selva.
Hardy, D. L., Sr. 1968. Male-male copulation
in captive
Mojave rattlesnakes (Crotalus scutulatus).
Bull. Chicago
Herp. Soc. 33(12):258-262.
Hardy, D. L., Sr. and J. Silva Haad. 1998. A
review of
venom toxinology and epidemiology of
envenoming of
the bushmaster (Lachesis) with report of a
fatal bite.
Bull. Chicago Herp. Soc. 33(6):113-123.
Hayes, W. K. and J. G. Galusha. 1984. Effects
of
rattlesnake envenomation upon mobility of
male wild and
laboratory mice. Bull. Maryland Herp. Soc.
20:135-144.
Huang, M. Z., P. Gopalakrishnakone, M. C.
Chung, and
R. M. Kini. 1997. Complete amino acid
sequence of an
acidic, cardiotoxic phospholipase A2 from the
venom of
Ophiophagus hannah (King Cobra): a novel
cobra venom
enzyme with “pancreatic loop”. Arch Biochem
Biophys
338, 150-156.
Hulin., A., Ochoa and J. M. Desbordes. 1982.
Envenimations par des crotalides en Guyane
Francaise.
Med. D’Afrique Noire 29:249- 225.
Kini, R. M., and Evans, H. J., 1992.
Structural domains
in venom proteins: evidence that
metalloproteinases and
nonenzymatic platelet aggregation inhibitors
(disintegrins)
from snake venoms are derived by proteolysis
from a
common precursor. Toxicon 30, 265-293.
Kondo, H., S. Kondo, S. Sadahiro, K.
Yamauchi, A.
Ohsaka, R. Murata. 1972. Estimation by a new
method
of the amount of venom ejected by a single
bite of
Trimeresurus species. Japan. J. Med. Sci.
Biol. 25:123
131.
Lucus, S. M., and Jurg Meier. 1995. The
biology and
distribution of spiders of medical
importance. In: J. Meier
and J. White, (eds.), Handbook of clinical
toxicology of
animal venoms and poisons. New York: CRC
Press.
Madsen, T. and R. Shine. 1992. A rapid,
sexually-selected
shift in mean body size in a population of
snakes.
Evolution. 46:1220-1224.
Magalhaes, A. and C.R. Diniz. 1979.
Purification and
partial characterization of the thrombin-like
enzyme from
the venom of Lachesis muta noctivaga. Toxicon
17,
Suppl. No. 1:112.
Magalhaes, A., R. N. Ferreira, M. Richardson,
S. Gontijo,
A. Yarleque, H. P. B. Magalhaes, C. Bloch, E.
F. Sanchez.
2003. Coagulant thrombin-like enzymes from
the venoms
of Brazilian and Peruvian bushmaster
(Lachesis muta
muta) snakes. Comparative Biochemistry and
Physiology
Part B 136:255-266.
Marsh, N.E., B. C. Whaler. 1984. The Gaboon
viper
(Bitis gabonica); its biology, venom
components and
toxinology. Toxicon 22 (5):669-94
�
Mellor, N. H. and J. C. Arvin. 1996. Case
report. A
bushmaster bite during a birding expedition
in lowland
southeastern Peru. Wildn. Environmental. Med.
3:236
240.
Milani Junior R, MT Jorge, FP de Campos, FP
Martins,
A Bousso, JL Cardoso, LA Ribeiro, HW Fan, FO
Franca,
IS Sano-Martins, D Cardoso, C Ide Fernandez,
JC
Fernandes, VL Aldred, MP Sandoval, G Puorto,
RD
Theakston and DA Warrell. 1997. Snake bites
by the
jararacucu (Bothrops jararacussu):
clinicopathological
studies of 29 proven cases in Sao Paulo
State, Brazil.
QJM, Vol 90, Issue 5 323-334, Oxford
University Press.
Minton, S. A. Jr. and M. R. Minton. 1969.
Venomous
reptiles. New York: Scribner.
Mitchell, S. W. 1860. Researches upon the
venom of the
rattlesnake. Smithson. Contrib. Knowledge
1860:1-139.
Pough, F. H. and J. D. Groves. 1983.
Specialization of
the body form and food habits of snakes. Am.
Zool.
23:443-454.
Reid, H. A. 1976. Venoms and Antivenoms.
(Review on
Glass, T. G. Jr. Early debridement in
pitviper bites.
J.A.M.A., 235:2513, 1976). Abstracts on
Hygiene
51:1139.
Ripa, D. 1994. Reproduction of the Central
American
bushmaster (Lachesis muta stenophrys) and the
black-
headed bushmaster (Lachesis muta
melanocephala) for
the first time in captivity. Bull. Chicago
Herp. Soc.
29(8):165-183.
Ripa, D. 1997. Range extension for Bothrops
leucurus.
Bull. Chicago Herp. Soc. 32(2):25-26.
Ripa, D. 1999. Keys to understanding the
bushmasters
(Genus Lachesis Daudin, 1803). Bull. Chicago
Herp.
Soc. 34(3):45-92.
Rosenfeld, G. 1971. Symptomatology,
pathology, and
treatment of snake bite in South America. Pp.
345-383.
In: W. Bucherl and E. E. Buckley, (eds.),
Venomous
animals and their venoms. Vol. 2. New York:
Academic
Press.
Rucavado, A., E. Flores Sanchez, A.
Franceschi, A.
Magalhaes, J. M. Gutierrez. 1999.
Characterization of
the local tissue damage induced by LHF-II, a
metalloproteinase with weak hemorrhagic
activity isolated
from Lachesis muta muta snake venom. Toxicon
37:12971312.
Russell, F. E. 1983. Snake venom poisoning.
Greatneck,
NY: Scholium International.
Sanchez, E. F., T. V. Freitas, D. L.
Ferreira-Alve, D. T.
Valverde, M. R. Diniz, M. N. Cordeiro,
G.Agostini-Cotta,
C. R. Diniz. 1992. Biological activities of
venoms from
South American snakes. Toxicon 30:95-103.
Sanchez, E. F., C. I, Santos, A. Magalhaes,
C. R. Diniz,
S. Figueiredo, J. Gilroy, M. Richardson.
2000. Isolation
of Proteinase with Plasminogen-Activating
Activity from
Lachesis muta muta (Bushmaster) Snake Venom.
Archives
of Biochemistry and Biophysics. Vol. 378, No.
1: 131141
Silva Haad, J. L. 1980/1981. Accidentes
humanos por
las serpientes de los generos Bothrops y
Lachesis. Mem.
Inst. Butantan 44/45:403-423.
Silva, L. M., C. R. Diniz and A. Magalhaes.
1985.
Purification and partial characterization of
an arginine
ester hydrolase from the venom of the
bushmaster snake,
Lachesis muta noctivaga. Toxicon 23(4): 707 -
718.
Solórzano, A. and L. Cerdas. 1986. A new
subspecies
of the bushmaster, Lachesis muta, from
southeastern
Costa Rica. J. Herpetology 20(3):463-466.
Solórzano, A. and L. Cerdas. 1989.
Reproductive biology
and distribution of the terciopelo, Bothrops
asper Garman
(Serpentes: Viperidae) in Costa Rica.
Herpetologica.
45(4):444-450.
Solórzano-López, A., M. Santana and H. W.
Greene. 1987.
Reproductive biology of Lachesis muta
stenophrys
(Serpentes: Viperidae) in Costa Rica. Abst.
Jt. Ann. Mtg.
SSAR Herpet. League :146.
Taylor, E. H. 1951. A brief review of the
snakes of Costa
Rica. Univ. Kansas Sci. Bull. 34:1-188.
Thomas, R. G. and F. H. Pough. 1979. The
effect of
rattlesnake venom on digestion of prey.
Toxicon 17:221
228.
Torres, J. R., M. A. Torres and M. A. Arroyo-
Parejo.
1995. Coagulation disorders in bushmaster
envenomation. [Letter] Lancet 346:449-450.
Vial, J. L. and J. M. Jiménez-Porras. 1967.
The
ecogeography of the bushmaster, Lachesis
muta, in
Central America. American Midland Naturalist.
78:182
187.
�
Watt, G. 1989. Snakebite treatment and first
aid. In: J.
Campbell and W. W. Lamar (eds.), The venomous
reptiles
of Latin America. Ithaca NY: Cornell
University Press.
White, J. 1995. Clinical toxicology of snake
bite in
Australia and New Guinea. In: J. Meier and J.
White,
(eds.) Handbook of clinical toxicology and
animal venoms
and poisons. New York: CRC press.
Yarleque, A. S. Campos, E. Escobar, F. Lazo,
N. Sanchez,
S. Hyslop, N. A. Marsh, P. J. Butterworth and
R. G.
Price. 1989. Isolation and characterization
of a
fibrinogen-clotting enzyme from venom of the
snake
Lachesis muta muta (Peruvian bushmaster).
Toxicon
27(11):1189-1197.
�
I'm sorry, this Article is unavailable or waiting for administration approval and therefore no comments are allowed.
|
Email Subscription
My Subscriptions
Subscriptions Help
Other Snake Bite Articles
 I Should Be Dead
I Should Be Dead
 Lachesis Bites in Brazil: 2 Cases
Lachesis Bites in Brazil: 2 Cases
 Snakebite in Sri Lanka
Snakebite in Sri Lanka
 Being Bit By the Big One
Being Bit By the Big One
|

